Get Support
+880-02-9337667SOMATEC PHARMACEUTICALS LTD. is a fast growing pharmaceutical company in Bangladesh.
28/B, Segun Bagicha, Dhaka-1000, Bangladesh.
+880-02-9337667
+880-02-9357827
+880-02-8391480
info@somatecpharmabd.com

The Manufacturing Plant is designed to meet cGMP standards as directed by WHO and offer flexibility in product lines and production volumes. The plant is designed to expand in a modular fashion through short notice.
With a firm commitment to produce highest quality Pharmaceuticals, the manufacturing facilities of SPL is well equipped and organized with most sophisticated machinery and equipment establishing state of the art facilities and are continuously upgrading and standardizing to meet the optimum level of international standards.
Somatec has created a strong differentiated product portfolio, building on its product development expertise, regulatory capabilities and large-scale production capacities with technological advancement.
Somatec believes in Total Quality Management (TQM) process which provides confidence, accuracy and strict compliance with current Good Manufacturing Practice (cGMP) as recommended by WHO and local regulatory authority that is founded on meeting expectation of customers and communities in which its employees live and work. We are committed to deliver medicines of outstanding quality.
Pharmaceuticals Product Development is challenging venture for local & global market and development of novel drug including biotechnological products. Constantly pumping of new products in market helps the company to survive and build up its image. Personnel of Product Development department always try to provide an opportunity to present the knowledge gained through the application of scientific approaches and quality risk management to the development of a product and its manufacturing process. It is important to recognize that quality cannot be tested into products, but should be built in the product by design. We introduce innovative high quality (efficacy & safe) new product considering cost effective. Our Product Development team emphasize on successful in developing & introducing quality products according to ICH guidelines and continuous improvement of new and existing product for customer satisfaction, environmental change, technology change and other competitors.
For that, we have dedicated and trained R&D personnel along with well-equipped separate Analytical laboratory with all necessary machineries and equipment of GMP standard in pilot scale.
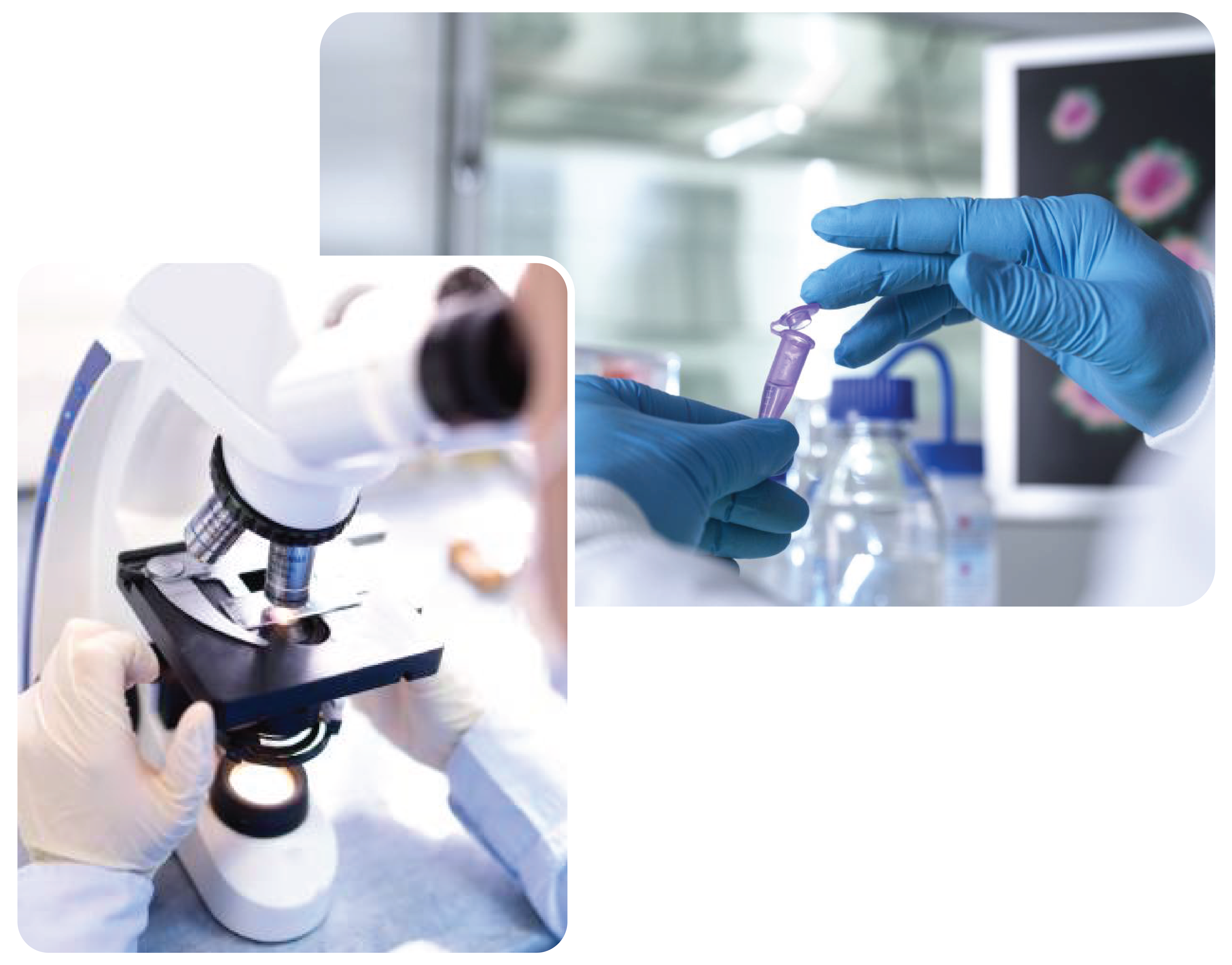

Our concern for quality is reflected in every aspect of product - from raw materials to finished products.
Quality Control is responsible for testing and analysis of drug substance, excipients, and packaging materials finished products, reagent management of QC Laboratory, reference standard management and preparation of testing instructions.
Our Quality Control Laboratory is equipped with highly sophisticated equipment including HPLC (High Performance Liquid chromatography), AAS (Atomic Absorption Spectrophotometer) TOC (Total Organic Carbon) Analyzer, UV-VIS Spectrophotometer, Automatic Potentiometer with Karl Fisher Titrator, FTIR (Fourier Transform infrared Spectrophotometer) etc. to conform “Good Laboratory Practice” (GLP) guideline so as to guarantee consistent product quality. We have got the machineries as follows:
In analytical and microbiological laboratories, young, skilled & competent professionals are working with a great sense of responsibility to ensure quality of every product.
Since SPL wishes to be a vital member to serve humanity, quality medicine is the only solution. Success in producing quality medicine largely depends on pivotal role of Quality Control Department.
Quality is the mainstay of our competitiveness. Our objective is to constantly achieve quality excellence. To achieve this, we take considerable focus in adherence to the QA policies. Quality Assurance, though an independent function, works as an interface between R&D and manufacturing strictly abiding with the standardized quality system, providing consistency, effectiveness and efficiency for all manufacturing activities across all our manufacturing locations. An excellent team of professionals having good understanding of concepts of quality systems and cGMP is working to ensure Quality Assurance at each stage right from receiving of starting materials to finished products to assure the highest international standards.
The major functions covered under the supervision of Quality Assurance:


The warehouse of SPL is a highly functional, utility building that protects the stored goods from environmental influences in an enclosed environment. This building is equipped with security measures against various disasters like fire and if any fire occurs the technical facilities of the building will allow the fire brigade to reach inside with appropriate fire-fighting equipment. And the locking system of the stored goods will ensure controlled access to the building. Specific quarantine and sampling areas with separate storage location allows the proper containment of cephalosporin and other sensitive materials. All the materials are stored in the warehouse by pallet racking. The status of materials and products are controlled by colored status stickers with four different storage conditions e.g.